AGLTS 2: beyond glove testing
STEQ America and Tema Sinergie presents the new automatic glove test system developed in response to the requirements of the new Annex 1
The name is AGLTS 2, and it’s the brand new automatic glove leak testing system designed for the pharmaceutical industry.
As the name suggests, AGLTS 2 is an evolution of the first automatic glove testing system developed by our partner Tema Sinergie, from which it inherits features, know how and stability. As a matter of fact though, it is definitely more than just an upgrade. AGLTS2 also features unique performances and characteristics that make it a cutting-edge system, responding to and anticipating the needs that the pharmaceutical industry will meet in the near future.
The development of this project was actually driven by two landmarks. On one hand, there’s the engineering experience, gained on the hundreds of units presently still active and used in several production sites; on the other, there’s the attention to the new needs of the pharmaceutical industry within the scenario designed by Annex 1.
The outcome is a new generation device way beyond the mere execution of glove testing, or supporting operators and pharmaceutical companies in the management of all glove testing related operations. All of this in full compliance with the most relevant regulations on the subject: Annex 1, EU Guidelines, GMP, ISO 14644 7 Annex E.5, Gamp5, FDA CFR 21 part 11.
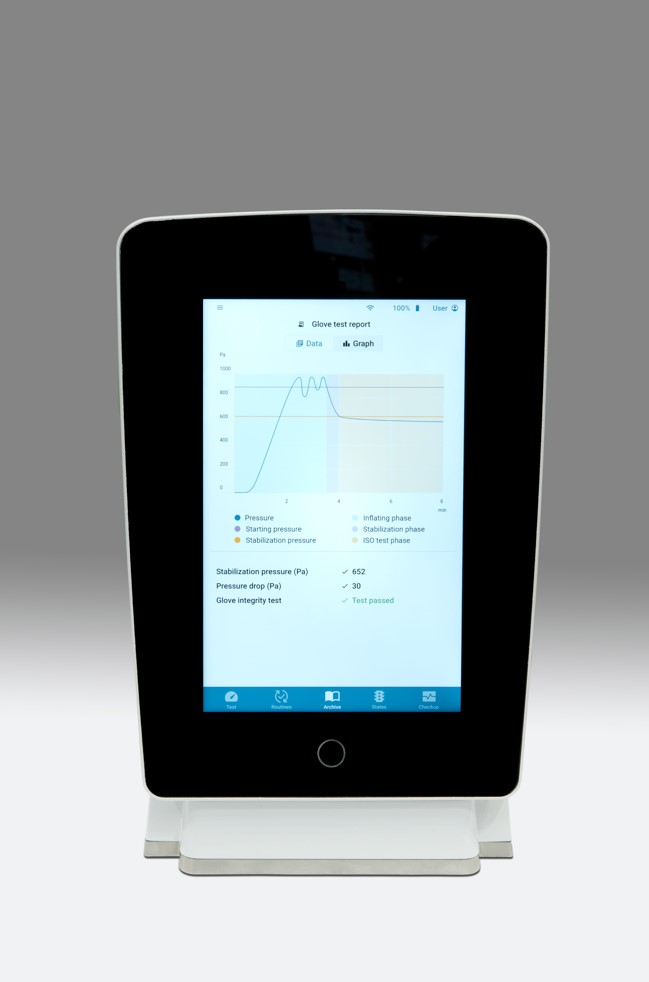
Beyond glove leak testing
Among the most interesting innovations introduced by AGLTS 2 in correlation to Annex 1 there’s certainly the Glove life cycle management, a feature providing the operator with a clear and wholesome view on all the data and parameters gathered on the tested gloves, the ones yet to be tested, and the whole life cycle of the glove itself. Such a time saving addition.
Yet another change which further identifies AGLTS2 is an on-board calibration and validation system, which improves the system reliability and remarkably decreases the chance for any possible false positive result, thus preventing the occurrence of problematic scenarios. This solution is extremely accurate, with no need for any calibration device or disruptive glove tests.
Some impressive news are also noticeable in terms of design and ergonomics. In detail, the integrated 10” touch screen giving access to an intuitive and user-friendly interface, even while wearing gloves. The screen also allows to access all of the reports on the device itself, in .pdf format.
Connection to corporate network has been improved as well. AGLTS2 may be integrated within the corporate network directly, via open LDAP protocol. This option gives the operators a chance to access AGLTS with the same credentials of the company network, in full compliance with FDA CFR 21 part 11.
Autonomy stays the same as in the previous version of the device. Just like its ancestor, AGLTS2 can perform a pressure drop integrity test compliant with ISO 14644-7 Annex E.5 automatically and without the need for external connections for electric or pneumatic power supply. This feature makes the use of AGLTS2 way easier in classified environments, compared to most of the systems currently available on the market.
In closing, AGLTS2 was designed to test all types of ports, as custom-made flanges can be assembled and installed, to grant high-level performances at all times.
The recognition of the door and the glove being tested takes place automatically, via Radio Frequency IDentification (RFID) Technology.
For more information feel free to contact us at info@steqamerica.com or phone 267-245-7010.
