The Challenges of Manually Cleaning Tanks and Blenders in Pharmaceutical Manufacturing – and How To Overcome Them
Introduction
If you work in a pharmaceutical production facility, you may be familiar with the customary objection – “I didn’t do it, why do I have to go in the tank?”. If you’re not familiar with this saying, you may be one of the fortunate ones who has not had to experience the manual cleaning of large tanks or blending equipment in all their magnificent confinement.
Manually cleaning bulk items is laborious, time-consuming, and potentially dangerous. Further difficulties may involve pressurized manual spraying and scrubbing/scraping, or immersion/flooding and rotating the equipment during cleaning, which may create accelerated deterioration with bearings and motors, because of the additional weight of the water continually circulating within the system. When operating equipment with various components, powdered materials can easily become entrapped in curves and crevices, risking future cross-contamination.A resounding obstacle nearly always at the forefront of commercial feasibility has been process cleaning and drying time. What if there was an approach to reduce this timeframe by a matter of hours?
An added question is also one of safety – having an employee placed inside of the equipment to hand-clean the residual debris that remains after production is completed. Then there’s the added task of finding and implementing a safe and suitable platform that can elevate these large pieces of heavy equipment to a point where cleaning and servicing can even begin safely and ergonomically.
 Powder Blending Concerns
Powder Blending Concerns
V-Blender shells, whether in an upright or vertical position, feature large access covers at the top of each of the v-ends (loading valves). The same is true of cone blender shells, where the cap sits at the bottom of the discharge port.
These covers are removed when loading powdered materials for blending. Once loading is completed, the covers are closed and the blending operations begin. This is where the process concern starts.
Powders are likely to not only become entrapped between the mating surfaces of the cover and the flange, but also amid the interior complex surfaces of the machinery itself. The potential of acquiring this impacted powder debris, together with additional powder amassing alongside this union during bending can be extremely problematic to clean. The loading valves on top of the blenders are an easily accessible location to manually clean, but the interior such as areas around baffles, ports, or discharge valves are unfortunately not so straightforward.
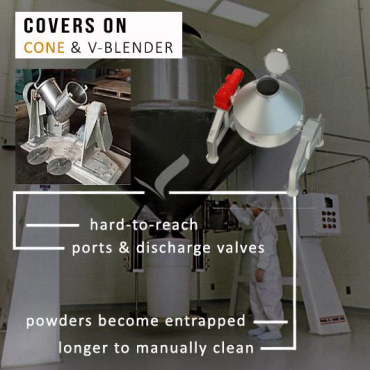
As different powders have variable viscosities and densities, they may blend well in production, but lead to issues when it comes to effective cleaning. During the blending cycle, the powders can may accumulate and amass into tight areas within the shell. Such sections which experience accumulation can be emptied during discharge, however if they are not cleared successfully, there is a heightened risk of failing to reach these areas when cleaning is then carried out manually.
It’s logical to ask oneself – “most powders are water-soluble, so can’t we just rinse them out?” Sometimes, this is easier said than done. Extra effort will undoubtedly be required for an infinitesimal percentage of the overall surface of the blender. But this may in turn reduce the optimal efficiency of the cleaning process.
It’s logical to ask oneself – “most powders are water-soluble, so can’t we just rinse them out?” Sometimes, this is easier said than done. Extra effort will undoubtedly be required for an infinitesimal percentage of the overall surface of the blender. But this may in turn reduce the optimal efficiency of the cleaning process.
Looking at Liquid Blending
Liquid process tanks are typically operated with a process skid, and a narrow system of pipework which functions as the conduit for product to transfer from the skid to the tank which is interconnected. The liquid that is supplied and drained through this restrictive piping therefore poses similar cleaning challenges to powder blenders.
When working with a liquid tank, you’re also dealing with the recurring risk of a potential build-up of organic material around the waterline. Just like the powder accumulation mentioned prior, this build-up around the waterline can require rigorous manual cleaning activity to eliminate.
Even the introduction of heated and chemically treated cleaning media across this dirty band of impacted material may not be enough to eradicate product buildup.
Cleaning Approaches: Good Vs. Bad
It may sound like a dated practice, but manual cleaning with a hose and brush is more common than most would like to think. For some facilities, it’s effective enough when performing inspections of cleanliness with the naked human eye, but can operators inspecting how well the equipment has been cleaned confidently make these independent and critical determinations?
Another approach is to immerse the equipment with water and cleaning chemicals before operating it. This can involve circulating cleaning fluid in the tank through a spray ball, or in the case of a blender, rotating it to create a vigorous movement of the cleaning media. Both methods may provide broad cleaning coverage on the surface but may not be sufficient for those harder to clean areas. Additionally, the water consumption involved in this method is significant. Not only does it become a culprit of increased operating costs because of the quality of purified and treated water needed which in itself is expensive, along with the sheer volume required, but this approach also costs the operator’s time.
All of these approaches are labor-intensive and routinely demand multiple operators. The disassembly of equipment prior to cleaning before the cleaning itself can even commence may add further valuable time to the whole cleaning process.
Operators may choose to extend the cleaning duration but this too doesn’t always resolve the issue, and instead may have an adverse effect when production time is delayed. In facilities where production turnover is already a holdup – extending the cleaning time just amplifies the dilemma. As they say, ‘desperate times call for desperate measures’, and it’s at this point a recommendation is often made to have an operator climb inside the vessel/tank to complete the cleaning – putting their safety in jeopardy. At what point does the production time of a product outweigh the safety concerns for an employee become acceptable?
Know What You’re Risking
There are strict standards that need to be upheld when it comes to cleaning validation. Some of the fundamentals ones include:
• Thoroughly remove all soil (manufacturing material) from all contact surfaces of the equipment to prevent future cross-contamination
• As much as possible, refrain from using potentially hazardous and corrosive cleaning chemicals to ensure these chemicals do not mix with production ingredients used to develop a more difficult to remove chemical mix in the future
Just as importantly, avoid putting operators in hazardous situations that could possibly cause them harm when manually cleaning equipment. This includes not only the physical risk of performing the task at hand, but also their exposure to dangerous product(s) or harmful cleaning agents.
What Can You Do?
That age-old saying “time is money” transcends across all industries and could not be more applicable when dealing with a manufacturing process in pharmaceutical environments. The faster equipment can be cleaned and turned over, the faster a new product batch can be produced and therefore more revenue generated. Process improvements in cleaning approaches have an immediate effect, and profitable long-term impact by paving the way for an overall boosted capacity.
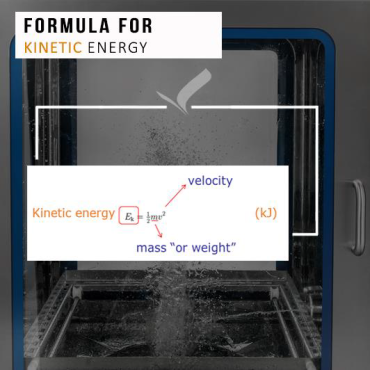 Replace manual spraying, scraping, brushing, recirculation of cleaning media and long contact times with a simple solution of kinetic energy (energy in motion equal to one half of the mass of the body, times the square of the velocity) and mechanical cleaning action by impingement (colliding or striking against the object).
Replace manual spraying, scraping, brushing, recirculation of cleaning media and long contact times with a simple solution of kinetic energy (energy in motion equal to one half of the mass of the body, times the square of the velocity) and mechanical cleaning action by impingement (colliding or striking against the object).
The principle of this solution is to use pressurized water and direct it at a surface with enough force to mechanically separate materials powerfully attached to the surface. 40lpm (10gpm) at 70bar (1,015psi) making contact with your soil or residual product material attached to a stainless-steel surface is potent enough to remove any traces of the matter.
Combining impingement cleaning with single pass water improves the process even further. Despite the water being discarded during the cleaning process the overall consumption is significantly reduced. This is accomplished by affecting 100% coverage of the inside of the tank or blender, typically in less than 2 minutes of process time.
There’s also no need for detergent use with this process change since the robust impingement action replaces the need for chemical degradation of the soil. This in turn eliminates any fear of cleaning chemical residue that could be left behind on equipment when manually cleaned.
Heat is an energy typically used in manual or circulated cleaning processes to aid in the chemical action and solubility technique, but heated media is also unnecessary in the impingement cleaning process because of the impingement system’s sheer force and direct effectiveness.
 An example of this cutting-edge system is the M-Line high-pressure washing solution. Not only is the M-Line completely mobile, but it’s also generally more affordable than other conventional Clean-in-Place (CIP) alternatives. This circulated CIP system incorporates heat exchangers and cleaning chemistry pumps.
An example of this cutting-edge system is the M-Line high-pressure washing solution. Not only is the M-Line completely mobile, but it’s also generally more affordable than other conventional Clean-in-Place (CIP) alternatives. This circulated CIP system incorporates heat exchangers and cleaning chemistry pumps.
The impact of the impingement and its ability to naturally cut through dirt also exceeds the need to use any detergent or silica or alumina particles in the water stream. By extracting heated media and cleaning chemistry from your cleaning procedure, the process is stripped down and less challenging to monitor and manage.
Low volume/high pressure impingement washing systems should be considered as the solution to an automated, repeatable, and validatable cleaning method.

Conclusion
Manual approaches to cleaning equipment in pharmaceutical manufacturing come with their own unique set of challenges. These processes are taxing, time-consuming, and worst of all – do not employ a validatable solution for those hard-to-clean areas. In the end, successful cleaning is only as good as the final, grimiest location, no matter how small, made clean to agreed inspection standards.
Risking the health and safety of an operator to clean a tank or blender is avoidable, especially when there are more optimal alternatives available which reduce the inherent dangers involved with more traditional manual cleaning methods. Using kinetic energy or a system exercising impingement action against a surface area for the removal of soil is a more effective solution that not only saves valuable production time, but decreases utility consumption and costs, and paves the way for today’s modern production facilities to focus on being successful and safe product manufacturers.
